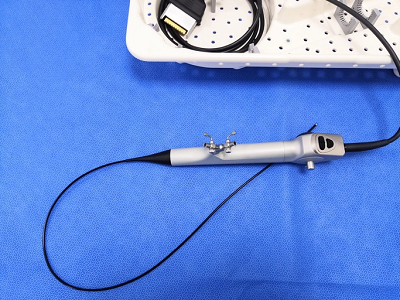
FDA warns of infection risk from reusable urological endoscopes
After receiving hundreds of reports over the last four years, the Food and Drug Administration is investigating whether reusable urological endoscopes pose an infection risk.
The devices allow clinicians to see or access the urinary tract, either to make a diagnosis or during a procedure.
Read more...